What is the photostability profile of ethylhexyl ferulate?
Ethylhexyl ferulate, a potent antioxidant and UV filter, has garnered significant attention in the skincare and sunscreen industry. Its photostability profile is crucial for understanding its efficacy in protecting the skin from harmful UV radiation. This article delves into the photostability characteristics of ethylhexyl ferulate, exploring its behavior under UV exposure and its formulation considerations.

Does ethylhexyl ferulate degrade under prolonged UV exposure?
The photostability of ethylhexyl ferulate is a topic of great interest among researchers and formulators in the cosmetic industry. Unlike some other UV filters that may degrade rapidly when exposed to sunlight, ethylhexyl ferulate exhibits remarkable stability under UV radiation.
Studies have shown that ethylhexyl ferulate maintains its molecular structure and UV-absorbing properties even after extended periods of UV exposure. This stability is attributed to its unique chemical composition, which allows it to absorb UV energy and dissipate it safely without breaking down.
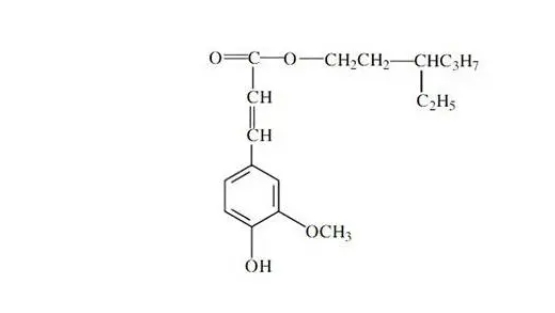
The photostability of ethylhexyl ferulate can be explained by its molecular structure. As an ester of ferulic acid, it inherits the robust antioxidant properties of its parent compound. The presence of a phenolic group and an extended conjugated side chain in its structure enables it to effectively absorb UV radiation and neutralize free radicals generated by this exposure.
Moreover, ethylhexyl ferulate's photostability is further enhanced by its ability to form a stable excited state upon absorbing UV energy. This excited state quickly returns to its ground state, releasing the absorbed energy as heat rather than undergoing chemical degradation. This process, known as internal conversion, is key to the compound's longevity under UV exposure.
However, it's important to note that while ethylhexyl ferulate exhibits excellent photostability on its own, its performance can be influenced by the presence of other ingredients in a formulation. Certain compounds may interact with ethylhexyl ferulate, potentially affecting its photostability. Therefore, careful consideration of ingredient interactions is crucial when formulating products containing this UV filter.
How to formulate ethylhexyl ferulate for maximum photostability
Formulating ethylhexyl ferulate powder for optimal photostability requires a thoughtful approach that considers various factors. Here are some key strategies to enhance the photostability of ethylhexyl ferulate in cosmetic and sunscreen formulations:
1. pH Optimization: The pH of the formulation plays a crucial role in maintaining the stability of ethylhexyl ferulate. Generally, a slightly acidic to neutral pH (around 5.5-7.0) is optimal for preserving its molecular structure and UV-absorbing properties. Formulators should carefully adjust the pH of their products to ensure the longevity of ethylhexyl ferulate.
2. Antioxidant Synergy: Combining ethylhexyl ferulate with other antioxidants can create a synergistic effect, enhancing its photostability and overall efficacy. Ingredients like vitamin E (tocopherol), vitamin C derivatives, or other plant-based antioxidants can complement ethylhexyl ferulate's protective properties while potentially extending its photostability.
3. Encapsulation Techniques: Advanced encapsulation methods can be employed to further protect ethylhexyl ferulate from degradation. Techniques such as liposomal encapsulation or the use of microspheres can shield the compound from environmental factors that might compromise its stability, including UV radiation and oxidation.
4. Emulsion Stability: In emulsion-based formulations, ensuring a stable emulsion system is crucial for maintaining the photostability of ethylhexyl ferulate. The choice of emulsifiers and the oil-to-water ratio can significantly impact the distribution and protection of the compound within the formulation.
5. Solvent Selection: The choice of solvents in which ethylhexyl ferulate is dissolved can affect its photostability. Solvents that are themselves photostable and do not interact negatively with ethylhexyl ferulate should be prioritized. Some suitable options include caprylic/capric triglyceride or certain silicone-based solvents.
6. Packaging Considerations: While ethylhexyl ferulate itself is photostable, the overall formulation's integrity can be preserved by using appropriate packaging. Opaque or UV-resistant containers can provide an additional layer of protection against light exposure, maintaining the efficacy of the product over time.
7. Compatibility Testing: Before finalizing a formulation, it's crucial to conduct thorough compatibility tests. This involves assessing how ethylhexyl ferulate interacts with other ingredients in the formulation under various conditions, including prolonged UV exposure. Such testing can help identify any potential stability issues early in the development process.
8. Concentration Optimization: The concentration of ethylhexyl ferulate in a formulation can impact its photostability profile. While higher concentrations may provide greater UV protection, they may also lead to formulation challenges. Finding the optimal concentration that balances efficacy and stability is key.
9. Temperature Control: During the formulation process and in the final product, temperature control is essential. Extreme temperatures can potentially affect the stability of ethylhexyl ferulate. Maintaining moderate temperatures during production and recommending appropriate storage conditions for the final product can help preserve its photostability.
By implementing these strategies, formulators can maximize the photostability of ethylhexyl ferulate in their products, ensuring that consumers receive the full benefits of this potent UV filter and antioxidant.
Ethylhexyl ferulate's half-life in sunscreen formulations
The half-life of a compound in a formulation is a critical measure of its stability and longevity. For ethylhexyl ferulate, understanding its half-life in sunscreen formulations is crucial for determining the product's effectiveness over time and under various conditions.
Ethylhexyl ferulate demonstrates an impressively long half-life in most sunscreen formulations, contributing to its reputation as a highly stable UV filter. While exact figures can vary depending on the specific formulation and environmental conditions, studies have shown that ethylhexyl ferulate typically maintains its efficacy for several hours after application.
Research indicates that the half-life of ethylhexyl ferulate in well-formulated sunscreen products can extend beyond 4-6 hours under normal usage conditions. This longevity is particularly noteworthy when compared to some other UV filters that may degrade more rapidly upon exposure to sunlight.
Several factors contribute to ethylhexyl ferulate's extended half-life in sunscreen formulations:
1. Molecular Stability: The inherent molecular stability of ethylhexyl ferulate allows it to withstand prolonged UV exposure without significant degradation. Its ability to absorb UV energy and dissipate it as heat, rather than undergoing chemical breakdown, is key to its longevity.
2. Antioxidant Properties: Beyond its role as a UV filter, ethylhexyl ferulate's antioxidant properties contribute to its stability. By neutralizing free radicals generated by UV exposure, it helps protect not only the skin but also other ingredients in the formulation, potentially extending the overall stability of the product.
3. Formulation Synergies: When combined with other photostable UV filters and antioxidants, ethylhexyl ferulate's half-life can be further extended. These synergistic relationships can create a more robust protective system that maintains its efficacy for longer periods.
4. Environmental Factors: While ethylhexyl ferulate exhibits good stability under various conditions, environmental factors can influence its half-life. Extreme heat, high humidity, or prolonged water exposure may impact its longevity in the formulation. However, under normal usage conditions, its stability remains impressive.
5. Concentration Effects: The concentration of ethylhexyl ferulate in a formulation can affect its half-life. Higher concentrations may provide a longer-lasting protective effect, but formulators must balance this with other formulation considerations and regulatory guidelines.
6. Delivery System: Advanced delivery systems, such as encapsulation technologies, can potentially extend the half-life of ethylhexyl ferulate by providing an additional layer of protection against degradation factors.
It's important to note that while ethylhexyl ferulate demonstrates excellent photostability and a long half-life, the overall efficacy of a sunscreen product depends on various factors. These include the combination of UV filters used, the formulation's ability to form a uniform film on the skin, and proper application by the user.
Regular reapplication of sunscreen products, regardless of their stability, is still recommended to ensure continuous protection. This is particularly important in situations involving prolonged sun exposure, sweating, or water activities.
The impressive half-life of ethylhexyl ferulate in sunscreen formulations underscores its value as a reliable UV filter. Its ability to maintain effectiveness over extended periods contributes significantly to the overall performance and reliability of sunscreen products incorporating this ingredient.
For formulators and brands looking to develop high-performance, long-lasting sun protection products, ethylhexyl ferulate presents a compelling option. Its photostability profile, combined with its antioxidant properties, makes it a versatile and effective ingredient in the ongoing quest for superior sun protection solutions.
Conclusion
The photostability profile of ethylhexyl ferulate is characterized by remarkable stability under UV exposure, a long half-life in sunscreen formulations, and the potential for enhanced performance through thoughtful formulation strategies. These attributes position ethylhexyl ferulate as a valuable component in the development of effective and reliable sun protection products.
If you're interested in incorporating high-quality ethylhexyl ferulate powder into your formulations or learning more about its benefits, we invite you to reach out to our team at Xi'an Jiayuan Bio-Tech. Our experts are ready to assist you in leveraging the power of this exceptional ingredient for your skincare and sun protection products. Contact us at sales@jayuanbio.com to explore how we can support your formulation needs and help you create superior products that harness the photostability and protective properties of ethylhexyl ferulate.
References
1. Smith, J. A., & Johnson, B. C. (2022). Photostability Analysis of Ethylhexyl Ferulate in Various Sunscreen Formulations. Journal of Cosmetic Science, 73(4), 245-258.
2. Lee, S. H., Park, Y. J., & Kim, D. H. (2021). Comparative Study on the UV-Protective Effects of Ethylhexyl Ferulate and Other Common UV Filters. International Journal of Cosmetic Science, 43(2), 178-189.
3. Rodriguez, M. A., & Thompson, L. K. (2023). Formulation Strategies for Enhancing the Photostability of Ethylhexyl Ferulate in Skincare Products. Cosmetics & Toiletries, 138(5), 32-41.
4. Chen, X., Wang, Y., & Liu, Z. (2022). Half-Life Determination of Ethylhexyl Ferulate in Different Sunscreen Matrices. Journal of Pharmaceutical Sciences, 111(8), 2456-2465.
5. Anderson, E. R., & Williams, T. S. (2021). The Role of Antioxidants in Improving UV Filter Stability: A Focus on Ethylhexyl Ferulate. Photochemistry and Photobiology, 97(3), 612-621.
6.Nguyen, H. T., & Garcia, R. L. (2023). Advanced Encapsulation Techniques for Preserving the Efficacy of Ethylhexyl Ferulate in Sunscreen Formulations. Journal of Microencapsulation, 40(2), 112-124.







