Pure Resveratrol Powder
CAS NO.: 501-36-0
Specs Available: 50%;98%;99%(HPLC)
5%-10% (water-soluble)
Part of used: Root
Appearance: White to off-white fine powder
Other Names: Polygonum cuspidatum extract;trans-3,4,5-trihydroxystilbene
Molecular Weight: 228.24
Molecular Formula: C14H12O3
Our Advantages: Scalable production capacity, strict quality control, cost efficiency from integrated factories, over 20 years of experience, advanced technology, and 24/7 after-sales support.
Certificaions: FSSC2000/ISO2000/HALAL/KOSHER/HACCP
Delivery terms: FedEx, DHL, EMS, UPS, TNT, all kinds of the airline, international shipping companies.
Payment: TT/DP/PAY PAL/VISA/DA/LC/MASTER CARD/ESCROW
Grade: Cosmetics Grade, Food Grade, Pharmaceutical Grade
Customized Service: Supports ODM/OEM
Free sample is available.
We do not sell retail quantities to individuals.
Product Introduction

Jayuan -Your Trusted Supplier of Resveratrol
Jayuan Bio is a high-tech enterprise that integrates scientific research, production, and sales. The company has self operated import and export rights, with a factory area of 12075 square meters, including a workshop area of 6520 square meters and a warehouse area of 2520 square meters. At the same time, we have five GMP compliant production lines, over 100000 square meters of self operated planting bases, and more than ten cooperative planting bases nationwide, achieving order based production from fields to enterprises. Our company has over 20 years of experience in the natural plant extract industry. Among them, resveratrol is one of our strongest products. We are one of the most professional manufacturers and suppliers of herbal extracts in China.
It is a natural polyphenolic compound found in plants such as grapes, blueberries, peanuts, and Polygonum cuspidatum. It has multiple health benefits, such as antioxidant, anti-aging, anti-inflammatory, cardiovascular protection, metabolic regulation, and neuroprotection.
Types of Resveratrol
Our company produces three types of the products: micronized resveratrol, resveratrol, and water-soluble resveratrol. We can flexibly provide cosmetic grade, food grade, and pharmaceutical grade of it according to customer needs, supporting one-stop services from sample testing to large-scale supply. In addition, we also support ODM/OEM customized services, providing personalized customization services for different customers to meet their needs to the greatest extent possible.
Different products have different production processes, so the prices are also different. If you are interested in our products, you can contact us via sales@jayuanbio.com and sales1@jayuanbio.com at any time if you feel free.
Prices for our Resveratrol:
Different specifications have different prices
Micronized Resveratrol
Resveratrol 99%
Water-soluble Resveratrol
Features of Micronized Resveratrol, Resveratrol 99%, and Water-Soluble Resveratrol
1. Micronized Resveratrol: We utilize advanced German-imported NETZSCH airflow milling equipment to achieve particle sizes below 3μm, significantly improving solubility and bioavailability (literature reports indicate particle sizes 2-5 times greater than those of standard powders). This ensures uniform, contamination-free product particles, achieving ultra-fineness control. Low-temperature milling technology effectively protects the active ingredients. Intelligent production utilizes real-time feed rate monitoring to ensure batch stability.

2. Resveratrol 99%: We utilize the latest synthetic biology technology to replace traditional plant extraction, combining supercritical CO₂ purification with AI-powered crystallization control to achieve a breakthrough process with a purity of ≥99.9% and a 40% cost reduction.
3. Water-Soluble Resveratrol: While it is readily soluble in organic solvents such as ethanol and methanol, it is insoluble in water, easily reacts with other chemicals, and exhibits poor light stability. We utilize hydroxypropy-β-cyclodextrin as a key excipient for our water-soluble resveratrol. Its water solubility is significantly better than that of natural cyclodextrin, effectively improving product solubility, bioavailability and stability.

Specification of Micronized Resveratrol
| Items | specifications |
| Appearance | White to light yellow fine powder |
| Purity | ≥ 98% (HPLC detection, common pharmaceutical/health grade) or ≥ 95% (food/cosmetic grade) |
| Molecular formula | C₁₄H₁₂O₃ |
| Molecular weight | 228.25 g/mol |
| Melting point | 253-255 ° C |
| Solubility | Slightly soluble in water, easily soluble in organic solvents such as ethanol and DMSO (improved water solubility after micronization) |
| Optical activity | No optical rotation (non chiral molecule) |
Specification of Resveratrol
| Items | specifications |
| Product Name | Resveratrol |
| CAS NO. | 501-36-0 (trans resveratrol) |
| Molecular formula | C₁₄H₁₂O₃ |
| Molecular weight | 228.25 g/mol |
| Structural type | Polyphenols (Astragalus compounds) |
| Natural sources | grape skins, tiger canes, blueberries, peanuts, etc |
Specification of Water-Soluble Resveratrol
| Items | specifications |
| Appearance | White to light yellow powder (or transparent liquid, depending on dosage form) |
| Solubility | Completely soluble in water (ordinary resveratrol has a water solubility of only 0.03 g/L), with a solubility of 1-10 g/L (25 ° C) |
| Purity (HPLC) | ≥ 95% (may contain carrier components after modification, please indicate the proportion of active ingredients) |
| PH value (1% solution) | 5.0-7.0 (neutral to weakly acidic, suitable for oral/cosmetic use) |
| molecular form | Trans resveratrol is predominant (with higher activity), and stability may be improved through encapsulation or derivatization |
Application Areas
- Dietary Supplements: It is a popular ingredient in dietary supplements aimed at promoting overall health and well-being.
- Cosmeceuticals: Its antioxidant properties make it a valuable addition to skincare formulations, helping combat signs of aging and protect against environmental stressors.
- Functional Foods: Resveratrol-enriched foods and beverages offer consumers a convenient way to incorporate this beneficial compound into their daily diet for added health benefits.
- Pharmaceuticals: Ongoing research into the therapeutic potential of it has spurred interest in its use in pharmaceutical applications, particularly in the areas of cardiovascular health and cancer prevention.

Here are three COA tables about resveratrol:
COA
1.Micronized Resveratrol
| Product Name: Micronized Resveratrol | |||
| Item | Specifications | Result | Method |
| Basic Product Information | |||
| Genus and Species | Pohygonum cuspidatum Sieb et Zucc. | Conform | / |
| Part of the Plant | Root | Conform | / |
| Country of Origin | China | Conform | / |
| Marker Compounds | |||
| Assay (Trans-resveratrol) | >99.0% | 99.76% | HPLC |
| Emodin | <0.07% | 0.02 | HPLC |
| Organoleptic Data | |||
| Appearance | Fine Powder | Conform | / |
| Color | Off-white to light yellow | Conform | / |
| Odor | Characteristic | Conform | / |
| Taste | Characteristic | Conform | / |
| Process Data | |||
| Method of Processing | Extraction | Conform | / |
| Solvent(s) Used | Ethanol & Water | Conform | / |
| Drying Method | Vacuum Drying | Conform | / |
| Excipient | None | Conform | / |
| Physical Characteristics | |||
| Solubility | soluble in Alcohol | Conform | Visual |
| Particles size analysis | d(10%) | 0.353μm | GBT 5507-2008 |
| d(50%) | 1.392μm | ||
| d(90%) | 2.951μm | ||
| Surface area mean diameter D (3,2) | 2.423μm | ||
| Volume mean diameter D (4,3) | 3.757μm | ||
| Size range | 0.059-112.0μm | ||
| Loss on drying | <1.0% | 0.51 | GB/T 14769-1993 |
| Ash Content | <1.0% | 0.48 | AOAC 942.05 18th |
| Solvent Residue | Eur Pharm(Alcohol) | 298ppm | GJ-QCS-1007 |
| Heavy Metals | |||
| Total Heavy Metals | <10ppm | Conform | USP <231> method II |
| As | <2.0ppm | Conform | AOAC 986.15, 18th |
| Pb | <2.0ppm | Conform | AOAC 986.15, 18th |
| Hg | <0.5ppm | Conform | AOAC 971.21, 18th |
| Cd | <0.5ppm | Conform | AOAC 971.21, 18th |
| Pesticide Residue | |||
| 666 | <0.2ppm | Conform | GB/T 5009.19-1996 |
| DDT | <0.2ppm | Conform | GB/T 5009.19-1996 |
| Microbiology | |||
| Total Plant Count | <1000 cfu/g | Conform | AOAC 990.12, 18th |
| Total Taste & Mold | <1000 cfu/g | Conform | FDA (BAM) Chapter 18, 8th Ed. |
| E Coli | Negative | Negative | AOAC 997.11, 18th |
| Salmonella | Negative | Negative | FDA (BAM) Chapter 5, 8th Ed. |
2.Resveratrol 99%
| Product Name: Resveratrol 99% | |||
| Item | Specifications | Result | Method |
| Appearance | Off-white to yellowish powder | Conform | Visual |
| IR | Spectrum must conform | Conform | IR |
| HPLC | Retention time must conform | Conform | HPLC |
| Moistue % | NMT0.5 | 0.09 | cp2020<0832> |
| Ash % | NMT0.5 | 0.08 | cp2020<2302> |
| Emodin % | NMT0.1 | None Detected | HPLC |
| Cis-resveratrol % | NMT0.1 | None Detected | HPLC |
| Single Impurity % | NMT0.1 | 0.02 | HPLC |
| Total Impurities % | NMT0.5 | 0.02 | HPLC |
| Purity % | 99.0MIN | 99.98 | HPLC |
| Heavy Metals ppm | NMT10 | Conform | ICP-MS |
| Lead ppm ppm | NMT0.5 | Conform | ICP-MS |
| Cadmium ppm | NMT1.0 | Conform | ICP-MS |
| Arsenic ppm | NMT1.0 | Conform | ICP-MS |
| Mercury ppm | NMT0.1 | Conform | ICP-MS |
| Particle size | 95% through 80-mesh sieve | 98.46 | cp2020<0982> |
| DBP ppm | NMT0.3 | Conform | LC-MS-MS |
| Residual solvents ppm (Ethanol) | NMT1000 | 234 | GC |
| Assay (on dry basis) | 99.0-101.5 | 99.38 | HPLC |
| Microbiology | <1 | cp2020<1105> | |
| Total Plate Count CFU/g | NMT1000 | <1 | cp2020<1105> |
| Yeast & CFU/g | NMT100 | ||
| E.Cloi, Salmonella and pseudomonss/g | Absent/10 | ND | cp2020<1106> |
| Coliform MPN/g | NMT0.92 | <0.3 | GB4789.3-2016 |
| Conclusion | This product complies with enterprise standard. | ||
3.Water-Soluble Resveratrol
| Product Name: Water-Soluble Resveratrol | |||
| Item | Specifications | Result | Method |
| Basic Product Information | |||
| Genus and Species | Pohygonum cuspidatum Sieb et Zucc. | Conform | / |
| Part of the Plant | Root | Conform | / |
| Country of Origin | China | Conform | / |
| Marker Compounds | |||
| Assay (Trans-resveratrol) | >10.0% | 11.56% | HPLC |
| Organoleptic Data | |||
| Appearance | Fine Powder | Conform | / |
| Color | Off-white to light yellow | Conform | / |
| Odor | Characteristic | Conform | / |
| Taste | Characteristic | Conform | / |
| Process Data | |||
| Method of Processing | Extraction | Conform | / |
| Solvent(s) Used | Ethanol & Water | Conform | / |
| Drying Method | Vacuum Drying | Conform | / |
| Carrier | Betacyclodextrin | Conform | / |
| Water Solubility | >0.08g/ml | Conform | |
| Ratio of Extraction | 100:1 | Conform | |
| Physical Characteristics | |||
| Solubility | soluble in Alcohol | Conform | Visual |
| Particles size analysis(80 mesh) | >98% pass 80 mesh | Conform | GBT 5507-2008 |
| Loss on drying | <2.0% | 0.68% | GB/T 14769-1993 |
| Ash Content | <1.0% | 0.54% | AOAC 942.05 18th |
| Solvent Residue | Eur Pharm(Alcohol) | 298ppm | GJ-QCS-1007 |
| Heavy Metals | |||
| Total Heavy Metals | <10ppm | Conform | USP <231> method II |
| As | <2.0ppm | Conform | AOAC 986.15, 18th |
| Lead | <2.0ppm | Conform | AOAC 986.15, 18th |
| Mercury | <0.5ppm | Conform | AOAC 971.21, 18th |
| Pesticide Residue | |||
| 666 | <0.2ppm | Conform | GB/T 5009.19-1996 |
| DDT | <0.2ppm | Conform | GB/T 5009.19-1996 |
| Microbiology | |||
| Total Plate Count | <1000 cfu/g | Conform | AOAC 990.12, 18th |
| Total Taste & Mold | <100 cfu/g | Conform | FDA (BAM) Chapter 18, 8th Ed. |
| E Coli | Negative | Negative | AOAC 997.11, 18th |
| Salmonella | Negative | Negative | FDA (BAM) Chapter 5, 8th Ed. |
Production process flow
This is our prodution process of Resveratrol. This process ensures the stability of product quality and solvent residue below international standards from raw materials to purification.

Granularity Report
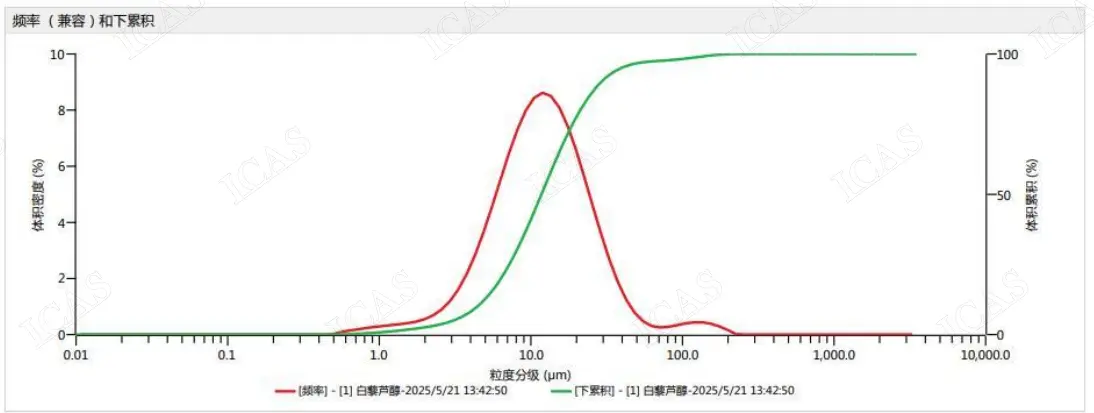
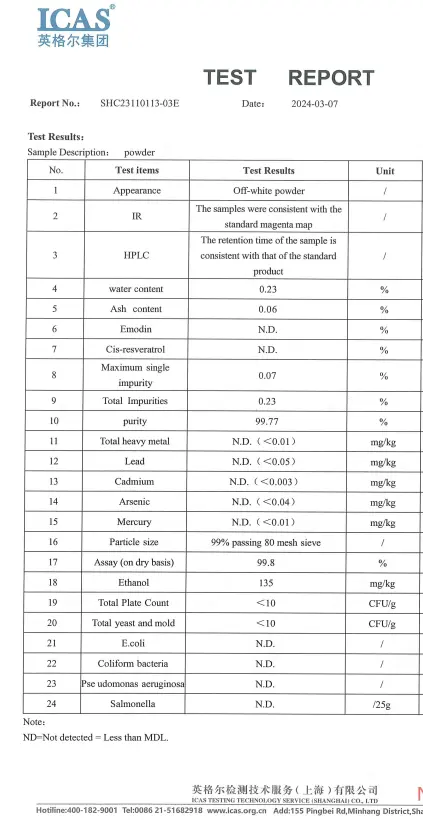
Test Report
Our product supports third-party testing to ensure high purity of product quality.
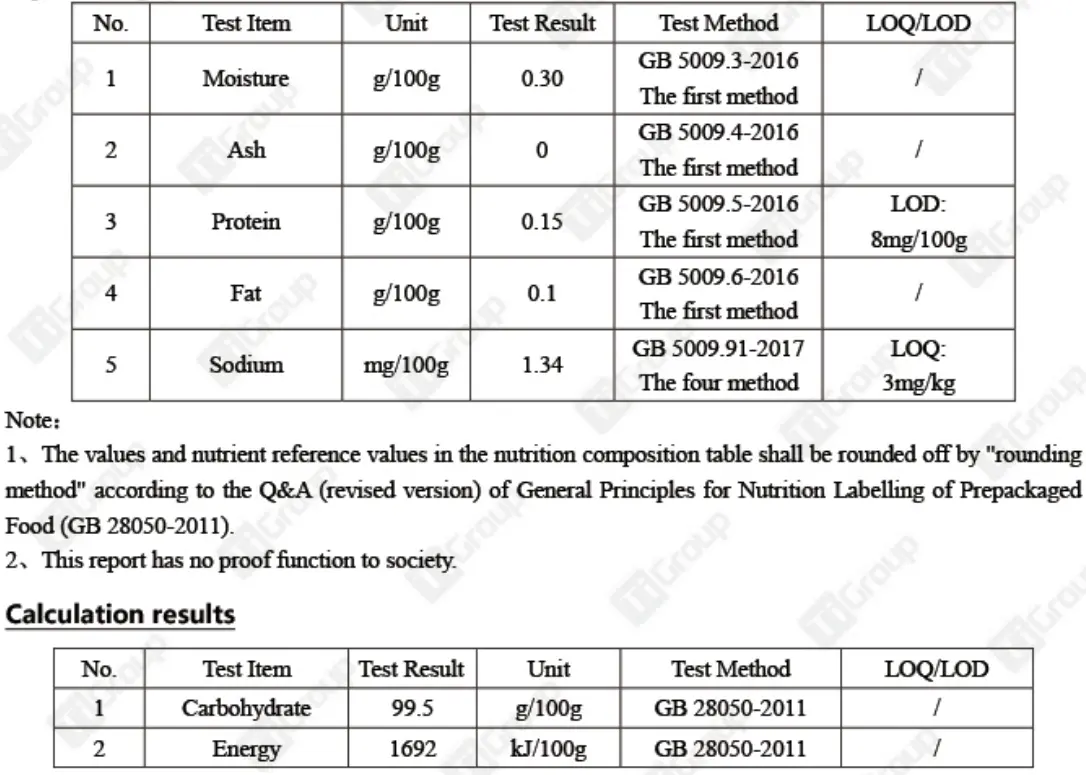
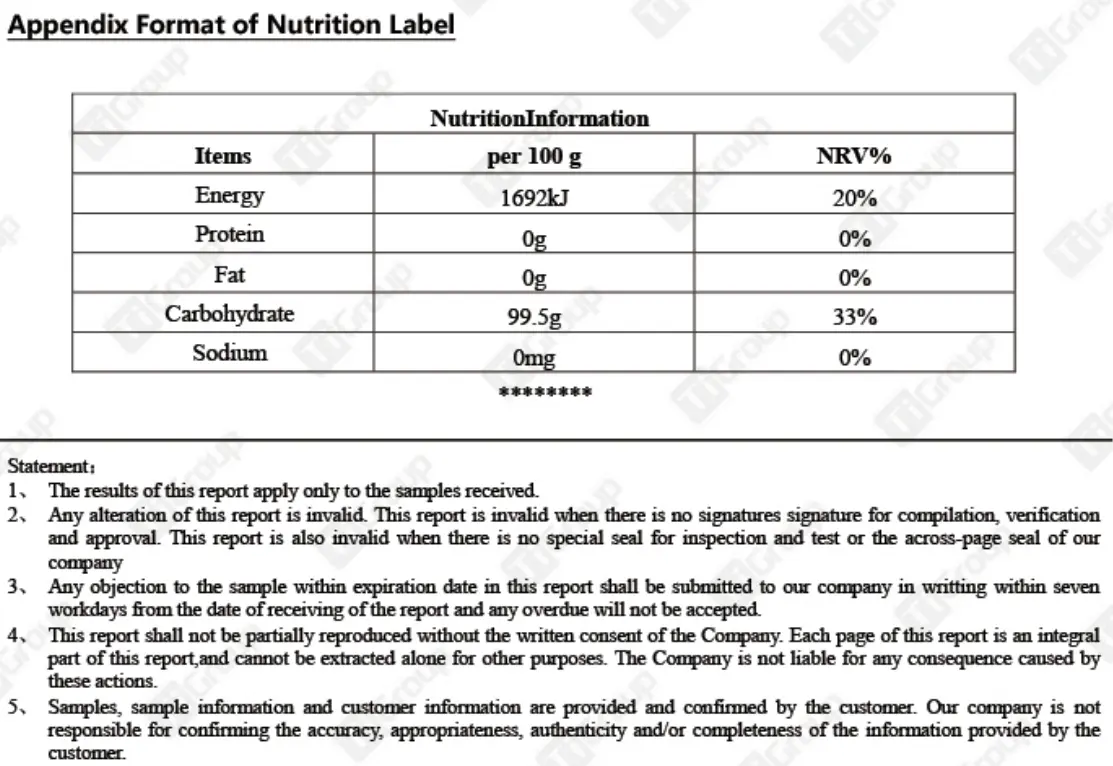
Supplement Facts
After the products we produce are released from the factory, they will be sent to third-party testing institutions for external inspection to ensure that the effective ingredients and nutritional components of the products are not missing.
Certificates
At Jayuan, we take pride in our commitment to quality and safety. Our product is manufactured in accordance with the highest industry standards and is certified by reputable organizations including FSSC22000, ISO22000, HALAL, KOSHER, and HACCP.

Patents
Jayuan has been deeply involved in the raw material medicine and plant extract industries for over 20 years, with a first-class R&D team and advanced equipment. We have obtained multiple patents.

Research and Development
Xi'an Jayuan Bio currently has over 80 employees and a professional R&D team of 30 people. Specializing in the research and testing of plant active ingredients, continuously improving and innovating extraction processes, standardized production processes, and a strong quality control management system, effectively ensuring the stability and bioavailability of product quality.

Transport & Package
We store it in aluminum foil bags or brown glass bottles in the dark, lined with polyethylene bags, sealed with nitrogen for effective moisture resistance, and refrigerated at 2-8 ℃ for long-term storage at -20 ℃. Effectively protect the stability of product performance. Support sea, land, and air delivery, with global reach, ensuring customers receive their goods as quickly as possible.

Exhibition Exchange
Jayuan has participated in industry exhibitions such as CPHI, API, PMEC, Vitafoods Asia for several consecutive years, engaging in in-depth exchanges and collaborations with pharmaceutical companies, research institutions, industry experts, and others from around the world on core products and the latest technological achievements. Through exhibition exchanges, Xi'an Jayuan Bio aims to showcase its technological strength and product advantages, expand domestic and international markets, seek more cooperation opportunities, and contribute to the development of the global pharmaceutical industry.
If you are interested in our products or would like to discuss cooperation matters, please contact us promptly. We will serve you wholeheartedly. I believe Jayuan will bring you a different experience and surprise.

Why Choose Us?
1. Full industry chain control: We have our own planting base and are equipped with advanced German imported airflow crushing equipment to ensure uniform and pollution-free product particles, achieve ultra-fine control, and effectively protect active ingredients with low-temperature crushing technology. Intelligent production monitors the feeding speed in real-time to ensure the stability of batch products. The residual heavy metals and solvents are below international standards, truly achieving integration from raw material cultivation to extraction and purification, reducing intermediate links, and ensuring full traceability of the production process, ensuring cost advantages and controllable prices. Provide customers with stable supply with high cost-effectiveness and support the delivery of large quantities of orders.
2. Standardized production process: We use organic solvent extraction method and select natural sources such as Polygonum cuspidatum and grape skins to ensure high initial active ingredient content (such as resveratrol glycoside in Polygonum cuspidatum ≥ 20%). Reduce physical impurities such as sediment and fibers through pre-treatment steps such as cleaning, drying, and crushing. Select the optimal ethanol with a food grade concentration of 60%~80% and a temperature of 50~70 ℃ (this method is safe, cost-effective, and has a high extraction rate). After concentrating the extract, determine the content of it by HPLC, and then detect ethanol residue by GC to meet ICH standards.

3. Multi stage purification: The first step is to concentrate the crude extract and remove most of the solvent by vacuum distillation. The second part of column chromatography purification uses macroporous adsorption resins (such as AB-8, HPD-100) or silica gel columns to separate the target components. Finally, crystallization and recrystallization are repeated using a solvent (such as methanol/water system) to remove impurities such as pigments and polysaccharides. Terminal purification using Agilent preparative HPLC for the final purification of pharmaceutical grade of it (≥ 99% purity). Using molecular distillation to separate thermosensitive impurities and improve purity. Use nanofiltration to remove trace amounts of macromolecular impurities (such as proteins and colloids). Step by step, effectively improving product purity and bioavailability.

4. Full process monitoring of the production process: Real time monitoring by HPLC, detecting the content of it in key steps such as extraction, concentration, and chromatography to ensure that the purity meets the standard at each stage (such as crude extract ≥ 50%, chromatography ≥ 90%). TLC thin layer chromatography can quickly screen for impurity spots. In terms of process parameter control, it is controlled from three aspects: pH value (such as pH 3-5 during extraction to prevent degradation), temperature (to avoid oxidation caused by high temperature), and pressure (precise regulation during supercritical fluid extraction). Effectively ensure product purity.

5. Impurity analysis and control: Regarding solvent residues, residual amounts such as ethanol and ethyl acetate were detected by GC (in accordance with ICH Q3C standards). For heavy metal residues, atomic absorption spectroscopy (AAS) or ICP-MS is used to detect lead, arsenic, etc. (≤ 10ppm). Pesticide residues are screened using GC-MS/LC-MS for organochlorine and organophosphorus pesticides (compliant with EU MRLs). Heavy metal and solvent residues are strictly controlled to the international pharmacopoeia standards (USP/EP), meeting the high-end demands of pharmaceuticals, health products, and cosmetics.
6. Professional testing report: We use patented purification technology, with a product purity of up to 99% and excellent batch stability performance. At the same time, key indicators such as heavy metals and solvent residues are strictly controlled, and the internal control standards of the enterprise are stricter than those in pharmacopoeias (such as USP/EP). For example, HPLC main peak area ≥ 98% (excluding solvent peaks), Single impurity≤ 0.5% (HPLC normalization method). Support third-party verification and send samples to SGS, Eurofins and other institutions for review. Simultaneously provide COA, heavy metal testing reports, and microbiological testing reports, SGS reports are provided with the goods, and support third-party testing. Realize full traceability from raw materials to finished products, ensuring batch quality stability and cost advantages. Assist customers in achieving maximum profit.

7. Professional testing equipment and personnel: We have five complete GMP standard production lines, a dedicated testing laboratory, and 20 professional testing personnel, providing full cooperation from the base to the workshop to the testing personnel. We are equipped with advanced Agilent and Waters high-performance liquid chromatography (HPLC) detection equipment, Agilent and Thermo Fisher gas chromatography (GC), Shimadzu and PerkinElmer ultraviolet spectrophotometers (UV), and Agilent and Waters mass spectrometry equipment for high-precision qualitative and quantitative analysis. Advanced instrument testing ensures high precision in product quality and purity.

8. Stability and packaging storage: Accelerated testing at 40 ℃/75% RH for 6 months, with regular detection of changes in content (degradation rate ≤ 5%). And long-term testing: 25 ℃/60% RH to determine the shelf life (usually 2-3 years). Store it in an aluminum foil bag or brown glass bottle away from light, lined with a polyethylene bag, sealed with nitrogen for effective moisture resistance, and refrigerated at 2-8 ℃ for long-term storage at -20 ℃. Effectively protect the stability of product performance.

9. Strong production capacity: Our high-quality product has a monthly output of 3-5 tons, with ton level inventory in stock to ensure timely delivery of large orders. We can provide one-stop service from sample testing to bulk supply.

10. Customized solutions: We provide customized packaging solutions such as ODM/OEM, supporting various specifications and forms of packaging needs, and flexibly meeting the usage scenarios of different customers.
11. Quality control: Our products have high purity, stable quality, and impurity control in accordance with EP/USP standards. They have also passed ISO9001 and ISO2000 quality management system certifications, and comply with food grade and cosmetic grade standards.

12. Logistics transportation: Global direct delivery by sea, land, and air, with a professional logistics team ensuring on-time delivery, covering markets in Europe, America, and Southeast Asia.

FAQ
Q1: Can I get free samples?
A: Certainly, we provide free samples.
Q2: Are there any discounts available?
A: Bulk purchases of quinoa protein powder come with discounts.
Q3: What is the minimum order quantity?
1 kilogram. Or contact us for more information.
Q4: What about delivery time?
A: Approximately 2-3 days after payment.
Contact Us
All in all, Resveratrol Extract Powder addresses a strong wellspring of cell reinforcements with boundless medical advantages. Whether integrated into dietary enhancements, skincare items, or utilitarian food sources, its flexibility and viability make it an important expansion to any health routine. At Jayuan, we are devoted to giving premium-quality unadulterated it, upheld by complete affirmations and prevalent client support. Join forces with us to open the maximum capacity of this momentous compound and take your items to a higher level.
Jayuan is a leading manufacturer and supplier of it, offering OEM and ODM services, extensive inventory, and complete certifications. With our one-stop standard service, fast delivery, and unwavering commitment to quality, we are your trusted partner in harnessing the power of it. Contact us at sales@jayuanbio.com and sales1@jayuanbio.com to learn more and embark on a journey towards healthier living.
You May Like
0


